
Chemistry, 19.04.2021 19:40 supasavb99
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to determine how many grams of aluminum oxide is formed during this reaction.
A.662.7 grams of Al2O3
B.24.6 grams of Al2O3
C.12.3 grams of Al2O3
D.6.1 grams of Al2O3
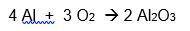

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
A 6.50-gram piece of aluminum reacts with an excess of oxygen. Use the balanced equation below to de...
Questions

English, 31.01.2020 16:56

History, 31.01.2020 16:56


Mathematics, 31.01.2020 16:56

Mathematics, 31.01.2020 16:56

Mathematics, 31.01.2020 16:56

History, 31.01.2020 16:56




History, 31.01.2020 16:56

Biology, 31.01.2020 16:56

History, 31.01.2020 16:56


Mathematics, 31.01.2020 16:56

History, 31.01.2020 16:56


Physics, 31.01.2020 16:56

Mathematics, 31.01.2020 16:56

Arts, 31.01.2020 16:56



