
Chemistry, 19.04.2021 21:00 student679
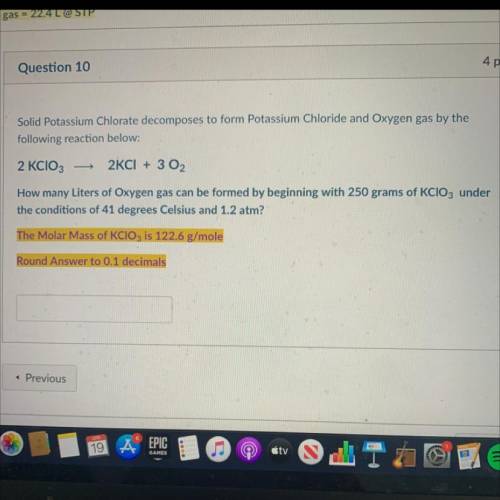
Solid Potassium Chlorate decomposes to form Potassium Chloride and Oxygen gas by the
following reaction below:
2 KCIO3
2KCI + 3 02
How many Liters of Oxygen gas can be formed by beginning with 250 grams of KCIO3 under
the conditions of 41 degrees Celsius and 1.2 atm?
The Molar Mass of KClO3 is 122.6 g/mole
Round Answer to 0.1 decimals

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1
You know the right answer?
Solid Potassium Chlorate decomposes to form Potassium Chloride and Oxygen gas by the
following reac...
Questions


Mathematics, 14.06.2021 18:40

Biology, 14.06.2021 18:40

Mathematics, 14.06.2021 18:40



Physics, 14.06.2021 18:40



Advanced Placement (AP), 14.06.2021 18:40

Mathematics, 14.06.2021 18:40






English, 14.06.2021 18:40



Computers and Technology, 14.06.2021 18:40



