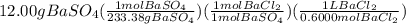Chemistry, 13.10.2019 10:20 luximartinez
Achemist wants to produce 12.00 grams of barium sulfate by reacting a .6000 m bacl2 solution with excess h2so as show in the reaction below. what volume of the barium chloride should be used?
bacl2 + h2so4 --> baso4 +2hcl

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
This is a mixture that has the same composition throughout.
Answers: 1

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

You know the right answer?
Achemist wants to produce 12.00 grams of barium sulfate by reacting a .6000 m bacl2 solution with ex...
Questions

Mathematics, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00

Computers and Technology, 26.06.2019 01:00

English, 26.06.2019 01:00



History, 26.06.2019 01:00

Social Studies, 26.06.2019 01:00


History, 26.06.2019 01:00

Mathematics, 26.06.2019 01:00

Chemistry, 26.06.2019 01:00

English, 26.06.2019 01:00


Physics, 26.06.2019 01:00

Chemistry, 26.06.2019 01:00

Advanced Placement (AP), 26.06.2019 01:00



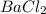
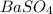 .
.