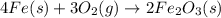Chemistry, 17.10.2019 07:30 deehunchoo
Iron metal reacts with oxygen gas to form rust, iron (iii) oxide. which of the following correctly shows the balanced equation for this reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2
You know the right answer?
Iron metal reacts with oxygen gas to form rust, iron (iii) oxide. which of the following correctly s...
Questions



Chemistry, 31.08.2021 03:10

English, 31.08.2021 03:10

Mathematics, 31.08.2021 03:10


Mathematics, 31.08.2021 03:10

History, 31.08.2021 03:10


Mathematics, 31.08.2021 03:10



Biology, 31.08.2021 03:10


Mathematics, 31.08.2021 03:10

Mathematics, 31.08.2021 03:10

English, 31.08.2021 03:10



Chemistry, 31.08.2021 03:10