
Calculate δh∘ in kilojoules for the reaction of ammonia nh3 (δh∘f=−46.1kj/mol) with o2 to yield nitric oxide (no) (δh∘f=91.3 kj/mol) and h2o(g) (δh∘f=−241.8kj/mol), a step in the ostwald process for the commercial production of nitric acid.
4nh3(g)+5o2(g)→4no(g)+6h2o(g)

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 23.06.2019 03:00
Use the half-reactions of the reaction au(oh)3 + hi -> au +i2 +h2o to answer the questions
Answers: 1
You know the right answer?
Calculate δh∘ in kilojoules for the reaction of ammonia nh3 (δh∘f=−46.1kj/mol) with o2 to yield nitr...
Questions

Biology, 28.06.2021 08:10


Social Studies, 28.06.2021 08:10

English, 28.06.2021 08:10


Mathematics, 28.06.2021 08:10


Computers and Technology, 28.06.2021 08:10

Mathematics, 28.06.2021 08:10

Mathematics, 28.06.2021 08:10

History, 28.06.2021 08:20

Mathematics, 28.06.2021 08:20

Biology, 28.06.2021 08:20

Mathematics, 28.06.2021 08:20



Mathematics, 28.06.2021 08:20

Mathematics, 28.06.2021 08:20

English, 28.06.2021 08:20

Mathematics, 28.06.2021 08:20

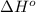 for the given reaction is -901.2 kJ.
for the given reaction is -901.2 kJ.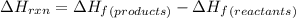
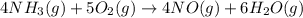
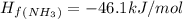
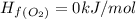
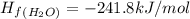
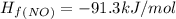
![\Delta H_{rxn}=[6\Delta H_f_{(H_2O)}+4\Delta H_f_{(H_2O)}-[4\Delta H_f_{(NH_3)}+5\Delta H_f_{(O_2)}]](/tpl/images/0307/6578/0d62c.png)
![\Delta H_{rxn}=[6mol(-241.8kJ/mol)+4mol(91.3kJ/mol)]-[4mol(-46.1kJ/mol)+5mol(0kJ/mol)]](/tpl/images/0307/6578/0673d.png)



