
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?
each bond in the molecule is only moderately polar covalent.
the molecule is too small to be polar.
the polar bonds are oriented in opposite directions.
the carbon atom has two unshared pairs of electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:10
56.16 gregor mendel was the first scientist to use statistics to analyze scientific data. before mendel's experiments, scientists believed that organisms acquired traits from their environment and passed them on to their offspring. after mendel's discoveries were accepted, scientists realized that traits passed to offspring were the result of genes being passed from parents to offspring. this is an example of pls
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Amolecule of carbon dioxide (co2) contains two polar covalent bonds. why is the molecule nonpolar?...
Questions


Chemistry, 18.06.2020 22:57


Mathematics, 18.06.2020 22:57

Mathematics, 18.06.2020 22:57




Mathematics, 18.06.2020 22:57






Mathematics, 18.06.2020 22:57



Mathematics, 18.06.2020 22:57


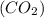 contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.
contains two polar covalent bonds. This molecule is non-polar because the polar bonds are oriented in the opposite direction.


