
Chemistry, 14.12.2019 12:31 mackenzie27717
Use vsepr theory to predict bond angles in the following covalently bonded molecules. explain your predictions.
a. methane
b. ammonia
c. water

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Use vsepr theory to predict bond angles in the following covalently bonded molecules. explain your p...
Questions

Social Studies, 19.11.2020 08:50

Mathematics, 19.11.2020 08:50

Arts, 19.11.2020 08:50

Social Studies, 19.11.2020 08:50


Mathematics, 19.11.2020 08:50

Business, 19.11.2020 08:50



Mathematics, 19.11.2020 08:50

Computers and Technology, 19.11.2020 08:50





Social Studies, 19.11.2020 09:00


Mathematics, 19.11.2020 09:00

Medicine, 19.11.2020 09:00

Mathematics, 19.11.2020 09:00

![Formula used :{\text{Number of electrons}} =\frac{1}{2}[V+N-C+A]](/tpl/images/0418/6428/ffc49.png)
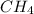
![Formula used :{\text{Number of electrons}} =\frac{1}{2}[4+4-0+0]=4](/tpl/images/0418/6428/2d0c5.png)
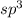 and geometry of the molecule will be tetrahedral.The bond angle for terahedral geometry is 109.5°.
and geometry of the molecule will be tetrahedral.The bond angle for terahedral geometry is 109.5°.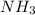
![Formula used :{\text{Number of electrons}} =\frac{1}{2}[5+3-0+0]=4](/tpl/images/0418/6428/13f6a.png)
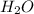
![Formula used :{\text{Number of electrons}} =\frac{1}{2}[6+2-0+0]=4](/tpl/images/0418/6428/1d091.png)



