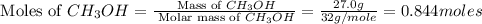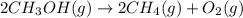Chemistry, 25.10.2019 18:43 samafeggins2
Consider the following reaction:
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amount of heat transferred when 27.0g of ch3oh(g) is decomposed by this reaction at constant pressure.
if someone could me with the steps i can figure it out on my own, you so much

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Consider the following reaction:
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amo...
2ch3oh(g)→2ch4(g)+o2(g)δh=+252.8kj< br /> calculate the amo...
Questions

Spanish, 09.12.2021 21:10

Mathematics, 09.12.2021 21:10


English, 09.12.2021 21:10

Mathematics, 09.12.2021 21:10

History, 09.12.2021 21:10


Social Studies, 09.12.2021 21:10




English, 09.12.2021 21:20


Mathematics, 09.12.2021 21:20





Social Studies, 09.12.2021 21:20

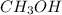 = 27.0 g
= 27.0 g