The acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many...

Chemistry, 30.08.2019 22:30 Lovely3719
The acetylene tank contains 35.0 mol c2h2, and the oxygen tank contains 84.0 mol o2.
how many moles of co2 are produced when 35.0 mol c2h2 react completely? how do you get 35.0 mol out of c2h2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 10:00
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

You know the right answer?
Questions

History, 27.08.2019 03:40

History, 27.08.2019 03:40


History, 27.08.2019 03:40

English, 27.08.2019 03:40

History, 27.08.2019 03:40

Chemistry, 27.08.2019 03:40

Mathematics, 27.08.2019 03:40

Health, 27.08.2019 03:40


Social Studies, 27.08.2019 03:40


History, 27.08.2019 03:40

History, 27.08.2019 03:40


English, 27.08.2019 03:40

Mathematics, 27.08.2019 03:40

Biology, 27.08.2019 03:40

Physics, 27.08.2019 03:40

Biology, 27.08.2019 03:40

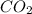 produced are, 70 moles
produced are, 70 moles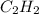 = 35 mole
= 35 mole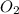 = 84 mole
= 84 mole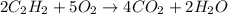
![\farc{4}{2]\times 35=70](/tpl/images/0213/2096/3d3b6.png) moles of carbon dioxide
moles of carbon dioxide

