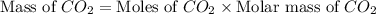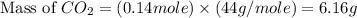You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bubbles as a reaction takes place. you then weigh the resulting solution and find that it has a mass of 64.96 g . the relevant equation is
caco3(s)+2hcl(aq)→h2o(l)+co2(g)+cac l2(aq)
assuming no other reactions take place, what mass of co2 was produced in this reaction?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1
You know the right answer?
You carefully weigh out 14.00 g of caco3 powder and add it to 56.70 g of hcl solution. you notice bu...
Questions

Mathematics, 30.01.2020 16:55


Health, 30.01.2020 16:55

Mathematics, 30.01.2020 16:55


Mathematics, 30.01.2020 16:55

Mathematics, 30.01.2020 16:55

Mathematics, 30.01.2020 16:55

Mathematics, 30.01.2020 16:55




Mathematics, 30.01.2020 16:55


Mathematics, 30.01.2020 16:55


Biology, 30.01.2020 16:55



Mathematics, 30.01.2020 16:55

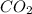 produced will be, 6.16 grams.
produced will be, 6.16 grams.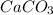 = 14 g
= 14 g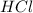 = 56.70 g
= 56.70 g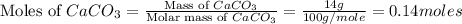
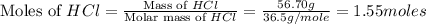
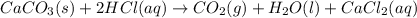
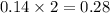 moles of
moles of