
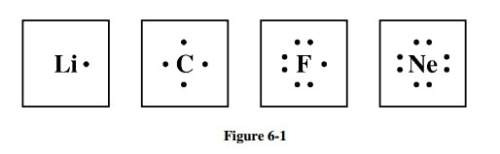
Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure 6-1. choose the statement that correctly identifies the most stable of the elements.
a. lithium is the most stable element because it has to lose only one electron to achieve a stable configuration.
b. carbon is the most stable element because it can form four bonds.
c. fluorine is the most stable element because it has to gain only one electron to achieve a stable configuration.
d. neon is the most stable element because its highest occupied energy level is filled.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Study the electron dot diagrams for lithium, carbon, fluorine, and neon in figure 6-1. choose the st...
Questions

Chemistry, 17.11.2020 22:00



Mathematics, 17.11.2020 22:00







History, 17.11.2020 22:00


English, 17.11.2020 22:00

Computers and Technology, 17.11.2020 22:00

Biology, 17.11.2020 22:00

Mathematics, 17.11.2020 22:00

Mathematics, 17.11.2020 22:00

Mathematics, 17.11.2020 22:10

Mathematics, 17.11.2020 22:10



