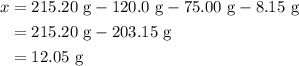Chemistry, 10.12.2019 23:31 sidallen05
When a solution of barium nitrate and a solution of copper (ii) sulfate are mixed, a chemical reaction produces solid barium sulfate, which sinks to the bottom, and a solution of copper (ii) nitrate. suppose some barium nitrate is dissolved in 120.00 g of water and 8.15 g of copper (ii) sulfate is dissolved in 75.00 g of water. the solutions are poured together, and a white solid forms. after the solid is filtered off, it is found to have a mass of 10.76 g. the mass of the solution that passed through the filter is 204.44 g. what mass of barium nitrate was used in the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2



Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
When a solution of barium nitrate and a solution of copper (ii) sulfate are mixed, a chemical reacti...
Questions



Mathematics, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30


English, 26.05.2021 22:30



Mathematics, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30


Mathematics, 26.05.2021 22:30

World Languages, 26.05.2021 22:30



Mathematics, 26.05.2021 22:30


Chemistry, 26.05.2021 22:30

Mathematics, 26.05.2021 22:30

 .
.
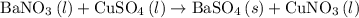
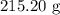 , and the mass of solid
, and the mass of solid 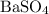 precipitate separated was
precipitate separated was 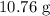 . Hence total mass of products is the sum of these two reactants.
. Hence total mass of products is the sum of these two reactants.
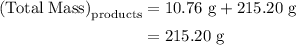
 . Mass of reactants involves mass of barium nitrate, water used for dissolving barium nitrate, mass of copper sulfate and water used for dissolving copper sulfate. The total mass of reactants is calculated as follows:
. Mass of reactants involves mass of barium nitrate, water used for dissolving barium nitrate, mass of copper sulfate and water used for dissolving copper sulfate. The total mass of reactants is calculated as follows:
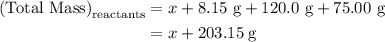
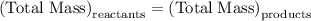 …… (1)
…… (1)
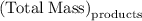 and
and 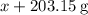 for
for 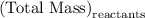 in equation
in equation 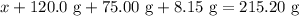
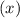 .
.