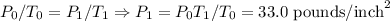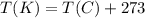Chemistry, 04.02.2020 06:51 sweetgirl22899
The pressure inside an an automobile tire is 44.0 pounds per square inch on a warm summer day (44.0 degree celcius). calculate the tire pressure in the same tire on a very cold day (-35.0 degree celcius) assuming that no air escapes from the tire

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
The pressure inside an an automobile tire is 44.0 pounds per square inch on a warm summer day (44.0...
Questions

Mathematics, 24.05.2021 20:00

English, 24.05.2021 20:00








Mathematics, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00


Biology, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00


Mathematics, 24.05.2021 20:00

Social Studies, 24.05.2021 20:00

Mathematics, 24.05.2021 20:00