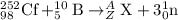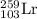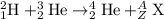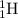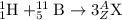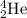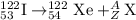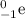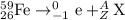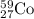Complete and balance the following nuclear equations by supplying the missing particle
a) 252/98cf + 10/5 b> 3 1/0n + ? (the 3 is separate from 1/0n, its not 31/0n)
b)2/1h + 3/2he > 4/2he + ?
c) 1/1h + 11/5b> 3?
d)122/53i(this is iodine) > 122/54 xe + ?
e)59/26fe. 0/-1e + ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Complete and balance the following nuclear equations by supplying the missing particle
a) 252...
a) 252...
Questions

Mathematics, 06.08.2021 04:40


Social Studies, 06.08.2021 04:40

Mathematics, 06.08.2021 04:40

Chemistry, 06.08.2021 04:40

Chemistry, 06.08.2021 04:40

Chemistry, 06.08.2021 04:40


English, 06.08.2021 04:50





Engineering, 06.08.2021 04:50

English, 06.08.2021 04:50


Chemistry, 06.08.2021 04:50


Mathematics, 06.08.2021 04:50