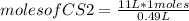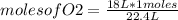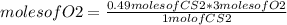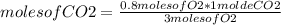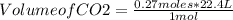Chemistry, 29.09.2019 16:30 lilpump3506
Identify the limiting reagent and the volume of co2 formed when 11l cs2 reacts with 18l o2 to produce co2 gas?
and so2 gas at stp.
cs2 (g) + 3o2(> co2(g) +2so2(g)
a.) o2; 6.0 l co2
b.)cs2; 11 l co2
c.) cs2; 5.5 l co2
d.) o2; 27 l co2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
Identify the limiting reagent and the volume of co2 formed when 11l cs2 reacts with 18l o2 to produc...
Questions


English, 10.01.2023 18:20









Mathematics, 11.01.2023 23:10

Mathematics, 12.01.2023 00:58



Social Studies, 13.01.2023 03:40

Mathematics, 13.01.2023 04:10


History, 13.01.2023 15:50

Mathematics, 13.01.2023 18:00