Chemistry, 07.10.2019 03:30 jacobp0712
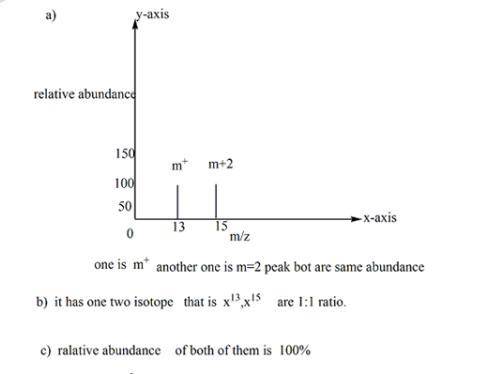
Suppose the mass spectrum of a hypothetical monatomic element x contains a signal at mass number 13 and another of identical height at mass number 15.
a. sketch the mass spectrum. make sure each axis is properly labeled.
b. how many isotopes are present? why?
c. what are the fractional abundances of the isotopes? why?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3


Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
Suppose the mass spectrum of a hypothetical monatomic element x contains a signal at mass number 13...
Questions




Health, 23.03.2020 16:41


Mathematics, 23.03.2020 16:41


History, 23.03.2020 16:41


Computers and Technology, 23.03.2020 16:42



Law, 23.03.2020 16:42







English, 23.03.2020 16:43