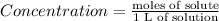Chemistry, 09.10.2019 15:10 marjorieheckaman
james created a salt water solution by adding a scoop of the solute, salt, to a beaker of the solvent, water. after all the salt dissolves, james places the beaker of salt water on a hot plate and begins to heat the solution. after half of the water evaporates, the concentration of the solution has
a) increased.
b) decreased.
c) stayed the same.
d) it is impossible to tell without measuring.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
james created a salt water solution by adding a scoop of the solute, salt, to a beaker of the solven...
Questions

Spanish, 05.07.2019 14:00









Mathematics, 05.07.2019 14:00

Social Studies, 05.07.2019 14:00


History, 05.07.2019 14:00






Physics, 05.07.2019 14:00

Mathematics, 05.07.2019 14:00