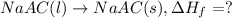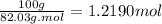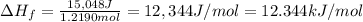Chemistry, 18.10.2019 12:00 christi1175
Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sodium acetate (naac) inside the calorimeter. when the sodium acetate finishes crystallizing, the temperature of the water inside the calorimeter is 32.2°c. the specific heat of water is
4.18 j/g-°c. what is the enthalpy of fusion (δhf) of the sodium acetate? show your work.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2
You know the right answer?
Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sod...
Questions


Mathematics, 12.01.2021 01:10

Social Studies, 12.01.2021 01:10

Mathematics, 12.01.2021 01:10





Mathematics, 12.01.2021 01:10

Geography, 12.01.2021 01:10



Social Studies, 12.01.2021 01:10

Biology, 12.01.2021 01:10

Mathematics, 12.01.2021 01:10


Mathematics, 12.01.2021 01:10



Social Studies, 12.01.2021 01:10

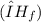 of the sodium acetate is 12.344 kJ/mol.
of the sodium acetate is 12.344 kJ/mol.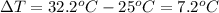
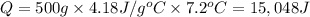
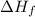 is the heat energy required to melt the one mole of substance.
is the heat energy required to melt the one mole of substance.