
Chemistry, 25.12.2019 15:31 sofiisabella10
Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given off in a reaction. suppose after five trials of a chemical reaction, 200 kj of heat was taken in on average. what conclusion can be made about the reaction? a. it is endothermic
.b. it releases heat energy.
c. it is a combustion reaction.
d. it creates more than one product.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:00
650.j is the same amount of energy as? 2720cal1550cal650.cal2.72cal
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
Me hurry
a calorimeter is a device used to measure how much heat energy is taken in or given...
a calorimeter is a device used to measure how much heat energy is taken in or given...
Questions

Mathematics, 19.05.2020 16:57





Physics, 19.05.2020 16:57

Chemistry, 19.05.2020 16:57


Chemistry, 19.05.2020 16:57

Computers and Technology, 19.05.2020 16:57





History, 19.05.2020 16:57


History, 19.05.2020 16:57



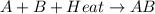
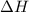 = +ve for an endothermic reaction.
= +ve for an endothermic reaction.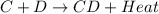 is an exothermic reaction.
is an exothermic reaction. 

