
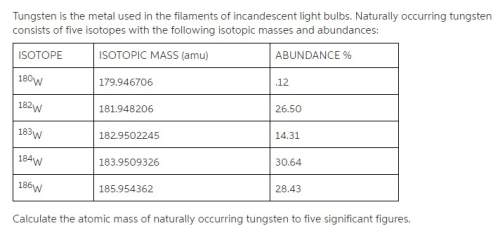
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungsten consists of five isotopes with the following isotopic masses and abundances:
calculate the atomic mass of naturally occurring tungsten to five significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1


Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

You know the right answer?
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungste...
Questions

















Computers and Technology, 12.08.2019 17:20






