Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:20
What would you do if you told the guy you liked that you liked him
Answers: 1

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2


Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
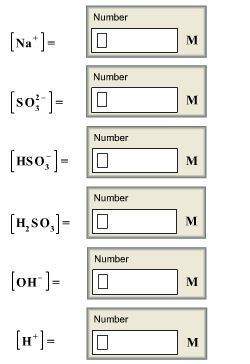
Calculate the concentrations of all species in a 1.70 m na2so3 (sodium sulfite) solution. the ioniza...
Questions


History, 19.07.2019 18:00








English, 19.07.2019 18:00

History, 19.07.2019 18:00

History, 19.07.2019 18:00


History, 19.07.2019 18:00

English, 19.07.2019 18:00

Mathematics, 19.07.2019 18:00

Mathematics, 19.07.2019 18:00

Mathematics, 19.07.2019 18:00

English, 19.07.2019 18:00

History, 19.07.2019 18:00