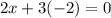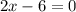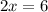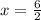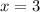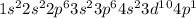Chemistry, 27.08.2019 10:30 ginareyes0423
In the 19th century, dmitri mendeleev predicted the existent of a then unknown element, x, with a mass of 68. he also predicted that an oxide of x would have the formula x2o3, on the modern period table. what is the group number and period of the element?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2
You know the right answer?
In the 19th century, dmitri mendeleev predicted the existent of a then unknown element, x, with a ma...
Questions


Mathematics, 23.07.2019 08:00

Mathematics, 23.07.2019 08:00


Mathematics, 23.07.2019 08:00


Computers and Technology, 23.07.2019 08:00



Mathematics, 23.07.2019 08:00


Social Studies, 23.07.2019 08:00

History, 23.07.2019 08:00


History, 23.07.2019 08:00



Mathematics, 23.07.2019 08:00

Social Studies, 23.07.2019 08:00

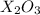 . From this, we can tell the charge of X. The charge of X is +3 and the charge of O is -2.
. From this, we can tell the charge of X. The charge of X is +3 and the charge of O is -2.