
Chemistry, 07.10.2019 08:02 ilovejustinbieber42
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the solubility product constant of cu3(aso4)2.
9.1 x 10^-4 m
3.4 x 10^-2 m
3.7 x 10^-8 m
8.7 x 10^-2 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2


Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the...
Questions

Biology, 27.11.2019 00:31




Mathematics, 27.11.2019 00:31






Mathematics, 27.11.2019 00:31

Social Studies, 27.11.2019 00:31






Physics, 27.11.2019 00:31

Mathematics, 27.11.2019 00:31

Mathematics, 27.11.2019 00:31

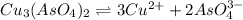
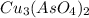 will be given by:
will be given by: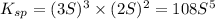
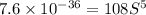
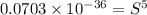
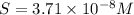
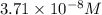 .
.

