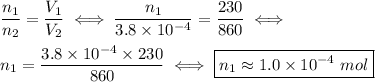Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Estructura 7.2 completar repaso 1 - ¿lógico o ilógico? 1 - ¿lógico o ilógico? listen and indicate whether each question and response is lógico or ilógico.
Answers: 3

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
You know the right answer?
Aballoon containing helium gas expands from 230 ml to 860 ml as more helium is added. what was the i...
Questions

Mathematics, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50


Mathematics, 02.03.2021 21:50

Health, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50



Mathematics, 02.03.2021 21:50


Chemistry, 02.03.2021 21:50


Biology, 02.03.2021 21:50

Mathematics, 02.03.2021 21:50


Mathematics, 02.03.2021 21:50



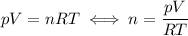
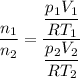
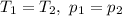 . Hence:
. Hence: