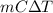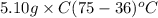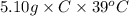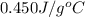Chemistry, 21.12.2019 17:31 nakarelinp0p303
A5.10 g sample of iron is heated from 36.0 to 75.0 c. the amount of energy required is 89.5 j. the specific capacity of this sample of iron
a.) 17800 j/g c
b.)0.900 j/g c
c.)11.7 j/g c
d.) 0.450 j/g c
e.) 2.22 j/g c

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

You know the right answer?
A5.10 g sample of iron is heated from 36.0 to 75.0 c. the amount of energy required is 89.5 j. the s...
Questions




History, 26.02.2020 16:43


Mathematics, 26.02.2020 16:43



Mathematics, 26.02.2020 16:43



Chemistry, 26.02.2020 16:43








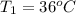 ,
,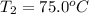 , q = 89.5 J
, q = 89.5 J