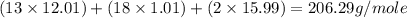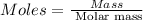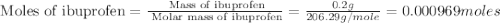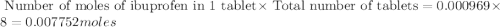1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet contains 200 mg of ibuprofen, and a typical adult dose is two tablets every six hours.
• determine the molar mass of ibuprofen. show all steps to find the answer.
• calculate the number of moles of ibuprofen in a single tablet. show all steps to find the answer.
• calculate the number of moles of ibuprofen that an adult would have taken if she took four doses of ibuprofen in one day. show all steps to find the answer.
asap need to get this done fast

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

You know the right answer?
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet...
Questions



Biology, 21.07.2019 21:30


History, 21.07.2019 21:30




Chemistry, 21.07.2019 21:30

Mathematics, 21.07.2019 21:30

Mathematics, 21.07.2019 21:30




History, 21.07.2019 21:30

Spanish, 21.07.2019 21:30

Mathematics, 21.07.2019 21:30



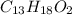 =
=