
Chemistry, 02.09.2019 07:10 Lindseycline123
What is the [h+] in a solution with poh of 0.253? 5.58 × 10−15 m 1.79 × 10−14 m 3.21 × 10−2 m 5.58 × 10−1 m

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2
You know the right answer?
What is the [h+] in a solution with poh of 0.253? 5.58 × 10−15 m 1.79 × 10−14 m 3.21 × 10−2 m 5.58...
Questions






Spanish, 28.04.2021 20:30



Mathematics, 28.04.2021 20:30

History, 28.04.2021 20:30






Mathematics, 28.04.2021 20:30

Computers and Technology, 28.04.2021 20:30

English, 28.04.2021 20:30


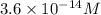
![pOH=-\log [OH^-]](/tpl/images/0219/8743/1fac1.png)
![0.253=-\log[OH^-]](/tpl/images/0219/8743/96531.png)
![[OH^-]=0.558M](/tpl/images/0219/8743/9d3a9.png)
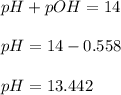
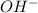 concentration.
concentration.
![pH=-\log [H^+]](/tpl/images/0219/8743/37e81.png)
![13.442=-\log [H^+]](/tpl/images/0219/8743/f459a.png)
![[H^+]=3.6\times 10^{-14}M](/tpl/images/0219/8743/7b6ee.png)
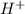 concentration is,
concentration is, 

