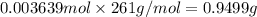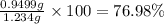The concentration of barium ions in any solution can also be determined via gravimetric analysis. an impure sample of barium nitrate with a mass of 1.234 g, is completely dissolved in water and the resulting solution is reacted with an excess of aqueous sodium sulfate. a precipitate forms, and after filtering and drying, it was found to have a mass of 0.848 g.
a) what is the relevance of adding eccess sodium sulfate?
b) calculate the % of barium nitrate in the original 1.234 g sample.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
The concentration of barium ions in any solution can also be determined via gravimetric analysis. an...
Questions


History, 09.12.2020 23:20




English, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20




Mathematics, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20


Mathematics, 09.12.2020 23:20


Biology, 09.12.2020 23:20

Mathematics, 09.12.2020 23:20


History, 09.12.2020 23:20

English, 09.12.2020 23:20

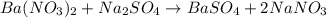
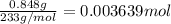
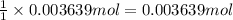 of barium nitrate.
of barium nitrate.