
Chemistry, 06.10.2019 10:00 kaylaamberd
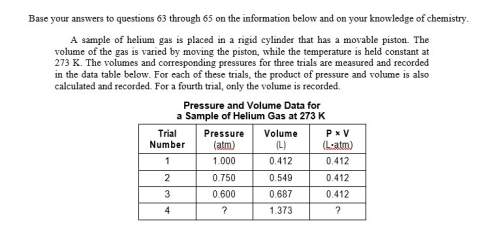
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas is varied by moving the piston, while the temperature is held constant at 273 k. the volumes and corresponding pressures for three trials are measured and recorded in the data table below. for each of these trials, the product of pressure and volume is also calculated and recorded. for a fourth trial, only the volume is recorded.
determine the pressure of the helium gas in trial 4. [1]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas...
Questions

Mathematics, 23.06.2019 04:31


Biology, 23.06.2019 04:31



Mathematics, 23.06.2019 04:31

Social Studies, 23.06.2019 04:31


Advanced Placement (AP), 23.06.2019 04:31

History, 23.06.2019 04:31

English, 23.06.2019 04:31

English, 23.06.2019 04:31

Mathematics, 23.06.2019 04:31

Advanced Placement (AP), 23.06.2019 04:31

Chemistry, 23.06.2019 04:31

Chemistry, 23.06.2019 04:31



Mathematics, 23.06.2019 04:31

English, 23.06.2019 04:31



