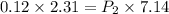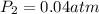Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2

Chemistry, 23.06.2019 17:50
How do chemical equations demonstrate conservation of mass
Answers: 1
You know the right answer?
If the pressure of the gas in a 2.31 l balloon is 0.12 atm and the volume increases to 7.14 l what w...
Questions

Mathematics, 07.05.2021 19:10

Mathematics, 07.05.2021 19:10



Mathematics, 07.05.2021 19:10

Mathematics, 07.05.2021 19:10



Mathematics, 07.05.2021 19:10

English, 07.05.2021 19:10

Mathematics, 07.05.2021 19:10



Arts, 07.05.2021 19:10

Health, 07.05.2021 19:10



Mathematics, 07.05.2021 19:10


Biology, 07.05.2021 19:10

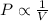 (At constant temperature and number of moles)
(At constant temperature and number of moles)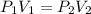
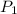 = initial pressure of gas = 0.12 atm
= initial pressure of gas = 0.12 atm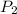 = final pressure of gas = ?
= final pressure of gas = ?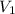 = initial volume of gas =
= initial volume of gas = 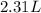
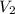 = final volume of gas = 7.14 L
= final volume of gas = 7.14 L