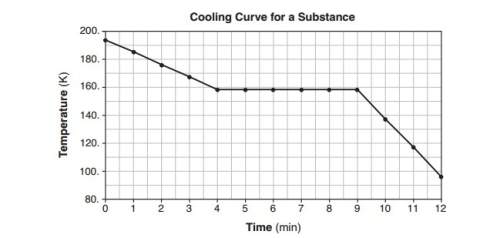
Abeaker contains a liquid sample of a molecular substance. both the beaker and the
liquid are...

Chemistry, 21.10.2019 15:30 averyeverdeen01
Abeaker contains a liquid sample of a molecular substance. both the beaker and the
liquid are at 194 k. the graph below represents the relationship between temperature and
time as the beaker and its contents are cooled for 12 minutes in a refrigerated chamber.
state what happens to the average kinetic energy of the molecules in the sample during
the first 3 minutes.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Questions


Chemistry, 19.06.2020 02:57




Mathematics, 19.06.2020 02:57


Mathematics, 19.06.2020 02:57


Biology, 19.06.2020 02:57





Mathematics, 19.06.2020 02:57







