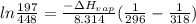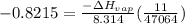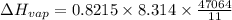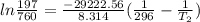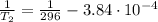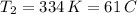Chemistry, 27.08.2019 03:30 lindseyr190
Chloroform, chcl3, was once used as an anesthetic. in spy movies it is the liquid put in handkerchiefs to render victims unconscious. its vapor pressure is 197 mmhg at 23 degrees c and 448 mmhg at 45 degrees
c. estimate its
a. heat of vaporization
b. normal boiling point i calculated the heat of vaporization to be 29.3 kj/mol. i'm having some trouble figuring out the normal boiling point, however. i know that the normal boiling point is when, at 1 atm, a liquid boils at a temperature at which its vapor pressure is equal to the pressure above its surface. so p1=p2=1 atm, if p1= vapor pressure and p2= atmospheric pressure/pressure above surface. i figured i could plug this into pv=nrt and solve, but i'm not given a lot of information. i considered assigning arbitrary values for n and v, so i would have
t= (1.00 atm)(1.00 l)/(1.00 ) but is that really the best way to do this problem, or would it even work at all?
i would think you could use the clausius-clapeyron equation, just as you did for delta h vap, but this time one of the ps will be 760 mm and calculate t for that p.
you, that makes sense, but how do i account for p2 and t2 in the equation if i don't know those values either?
but you have two vapor pressures at two temperatures. i would pick 23 c (change to kelvin, of course) and 197 mm for t1 and p1. then 760 mm and t2 for the others. you have all of the other numbers. check my thinking.
oh, of course, i had completely forgotten about that. you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Chloroform, chcl3, was once used as an anesthetic. in spy movies it is the liquid put in handkerchie...
Questions





History, 11.07.2019 08:40

Mathematics, 11.07.2019 08:40

Mathematics, 11.07.2019 08:40


History, 11.07.2019 08:40

English, 11.07.2019 08:40

Chemistry, 11.07.2019 08:40

English, 11.07.2019 08:40






English, 11.07.2019 08:40

Mathematics, 11.07.2019 08:40


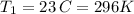
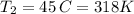
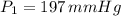
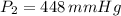
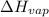 : heat of vaporization
: heat of vaporization