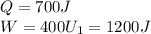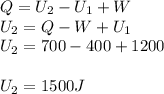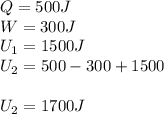Chemistry, 23.12.2019 07:31 Victoriag2626
In a heat engine, if 700 j of heat enters the system, and the piston does 400 j of work, what is the final internal (thermal) energy of the system if the initial energy is 1,200 j?
1,100 j
900 j
1,500 j
300 j
in a heat engine, if 500 j of heat enters the system, and the piston does 300 j of work, what is the final internal (thermal) energy of the system if the initial energy is 1,500 j?
1,700 j800 j1,300 j200 j

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2


Chemistry, 22.06.2019 19:00
Imagine that a new planet is discovered with two moons of equal mass: moon a and moon b. the mass of the new planet is greater than the combined mass of its moons. moon a is farther away from the new planet than moon b. what is the planet's gravitational pull on moon a compared to the planet's gravitational pull on moon b? the planet's gravity repels moon a with a greater force than it repels moon b, which is why moon a is farther away. the gravitational pull on moon b is greater than on moon a because moon b is closer to the new planet than moon a. the gravitational pull on moon b is greater than on moon a because moon b is farther away from the new planet than moon a. the gravitational pull on moon a is the same as the gravitational pull on moon b because distance does not affect the planet's gravity.
Answers: 1

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
In a heat engine, if 700 j of heat enters the system, and the piston does 400 j of work, what is the...
Questions

Physics, 23.10.2021 03:10





Social Studies, 23.10.2021 03:10





Chemistry, 23.10.2021 03:10

History, 23.10.2021 03:10


English, 23.10.2021 03:10




English, 23.10.2021 03:10