If the ph value was 3.6, what is the [h+]?
2.5 x 10-4
4.0 x 103...

Chemistry, 31.01.2020 08:51 afosburgh20
If the ph value was 3.6, what is the [h+]?
2.5 x 10-4
4.0 x 103

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:10
Abullet found at a crime scene may be used as evidence in a trial if the percentage of metals match to the composition of metals in a bullet from the suspect's ammunition. a forensic scientist's analysis of the bullet shows that it contains 11.9 g of lead, 0.5 g of tin, and 0.8 b of antimony. what is the percentage of lead metal in the bullet? express your answers to the one's place.
Answers: 2

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
Questions

History, 22.11.2020 01:00

Mathematics, 22.11.2020 01:00

Mathematics, 22.11.2020 01:00



Mathematics, 22.11.2020 01:00


English, 22.11.2020 01:00




Mathematics, 22.11.2020 01:00

Mathematics, 22.11.2020 01:00


Mathematics, 22.11.2020 01:00

Social Studies, 22.11.2020 01:00


English, 22.11.2020 01:00


History, 22.11.2020 01:10

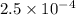
![pH=-\log[H^+]](/tpl/images/0489/1464/cf945.png)
![3.6=-\log[H^+]](/tpl/images/0489/1464/6e0d3.png)
![[H^+]=2.5\times 10^{-4}](/tpl/images/0489/1464/34a72.png)


