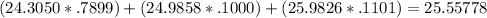Chemistry, 02.02.2020 22:56 RockieLuv7292
Magnesium has three naturally occurring isotopes: mg-24 with mass 24.3050 amu and a natural abundance of 78.99 %, mg-25 with mass 24.9858 amu and a natural abundance of 10.00 %, and mg-26 with mass 25.9826 amu and a natural abundance of 11.01 %.
calculate the atomic mass of magnesium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aspirin has a density of 1.40 g/cm3 what is the volume in cubic centimeters of a tablet weighing 320 mg ?
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Magnesium has three naturally occurring isotopes: mg-24 with mass 24.3050 amu and a natural abundan...
Questions


English, 27.06.2019 18:30









Social Studies, 27.06.2019 18:30


Mathematics, 27.06.2019 18:30

Biology, 27.06.2019 18:30

History, 27.06.2019 18:30