
Determine the concentrations of k2so4, k , and so42– in a solution prepared by dissolving 1.80 × 10–4 g k2so4 in 1.50 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm). note: determine the formal concentration of so42–. ignore any reactions with water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Determine the concentrations of k2so4, k , and so42– in a solution prepared by dissolving 1.80 × 10–...
Questions









Mathematics, 01.08.2020 08:01

Health, 01.08.2020 08:01







Biology, 01.08.2020 08:01

Biology, 01.08.2020 08:01


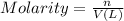
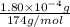
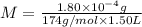
![[K_2SO_4]=M=6.8965\times 10^{-7} mol/L](/tpl/images/0431/3131/1bbe3.png)
![[K^+]=2\times M=2\times 6.8965\times 10^{-7} mol/L=1.3793\times 10^{-6} mol/L](/tpl/images/0431/3131/f96a0.png)
![[SO_4^{2-}]=1\times 6.8965\times 10^{-7} mol/L=6.8965\times 10^{-7} mol/L](/tpl/images/0431/3131/472ff.png)
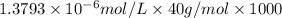
![[K^+]=0.05517 ppm](/tpl/images/0431/3131/6868b.png)
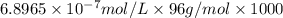
![[SO_4^{2-}]=0.06620 ppm](/tpl/images/0431/3131/956d0.png)


