Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
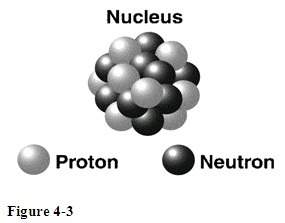
What is the change on the subatomic particles represented in figure 4-3? assuming all the particles...
Questions

Mathematics, 07.03.2021 09:50


Physics, 07.03.2021 09:50



Spanish, 07.03.2021 09:50






Social Studies, 07.03.2021 09:50



Mathematics, 07.03.2021 09:50


Mathematics, 07.03.2021 09:50

Mathematics, 07.03.2021 09:50


Mathematics, 07.03.2021 09:50