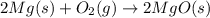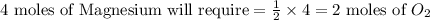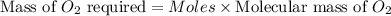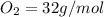Chemistry, 23.01.2020 14:31 blessed4628
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium oxide (mgo).
2mg + o2 mc009-1.jpg 2mgo
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 is required to react completely with 4.00 mol of mg?
2.00

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1


Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 04:00
Calculate the mass of 0.750 mol of the following substance. na3po4. , i'm not quite sure on how to set up the problem to solve! : (
Answers: 1
You know the right answer?
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium o...
Questions

Mathematics, 29.08.2019 18:00

Mathematics, 29.08.2019 18:00

Mathematics, 29.08.2019 18:00





History, 29.08.2019 18:00

Chemistry, 29.08.2019 18:00


English, 29.08.2019 18:00









Biology, 29.08.2019 18:00

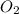 will be required to react completely with 4 moles of Mg.
will be required to react completely with 4 moles of Mg.