
Chemistry, 29.01.2020 00:52 waterdrop026
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at 25.0 ∘ c . a reaction vessel initially contains 250.0 ml of 0.104 m ab which is allowed to react to form the gaseous product. the product is collected over water at 25.0 ∘ c . part a how much time is required to produce 142.0 ml of the products at a barometric pressure of 707.3 mmhg . (the vapor pressure of water at this temperature is 23.8 mmhg .)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at...
Questions



Mathematics, 23.09.2021 14:00


English, 23.09.2021 14:00



Mathematics, 23.09.2021 14:00



Mathematics, 23.09.2021 14:00

Mathematics, 23.09.2021 14:00



Mathematics, 23.09.2021 14:00



Health, 23.09.2021 14:00


![v=k[AB]^2](/tpl/images/0479/4220/bde1b.png) . In the statement, we obtain that
. In the statement, we obtain that ![[AB]=0.104~M](/tpl/images/0479/4220/4f567.png) and, at 25 ºC,
and, at 25 ºC, 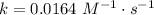 . Then:
. Then:![v=k[AB]^2\\\\ v=0.0164\cdot0.104^2\\\\ v=0.0164\cdot0.010816\\\\ v\approx0.000177=1.77\times10^{-4}~mol/s](/tpl/images/0479/4220/68b4c.png)


 . Hence:
. Hence: 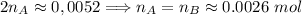 .
. . Furthermore, since the ratio of AB to A and to B is 1:1,
. Furthermore, since the ratio of AB to A and to B is 1:1,  .
.![v=\dfrac{|\Delta[AB]|}{\Delta t}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_{AB}|}{V}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_A||}{250~mL}\\\\ 1.77\cdot10^{-4}=\dfrac{1}{\Delta t}\cdot\dfrac{0.0026~mol}{0.25~L}\\\\ \Delta t=\dfrac{0.0026}{0.25\cdot1.77\cdot10^{-4}}\\\\ \boxed{\Delta t\approx58.76~s}](/tpl/images/0479/4220/8e656.png)


