Which statement best describes a bronsted-lowry base?
a) it must accept protons to form a con...

Chemistry, 31.01.2020 23:01 jaymoney0531
Which statement best describes a bronsted-lowry base?
a) it must accept protons to form a conjugate base.
b) it must accept protons to form a conjugate acid.
c) it must donate protons to form a conjugate base.
d) it must donate protons to form a conjugate acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

You know the right answer?
Questions


Biology, 26.02.2020 07:52

Mathematics, 26.02.2020 07:52

Business, 26.02.2020 07:53



Mathematics, 26.02.2020 07:53

Biology, 26.02.2020 07:53

Business, 26.02.2020 07:53






Mathematics, 26.02.2020 07:53



Mathematics, 26.02.2020 07:54

Mathematics, 26.02.2020 07:54

Mathematics, 26.02.2020 07:54

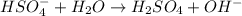
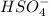 is a Bronsted-Lowry base because it can accept proton or hydrogen ion from
is a Bronsted-Lowry base because it can accept proton or hydrogen ion from 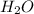 .
. 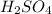 as conjugate acid.
as conjugate acid.

