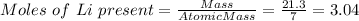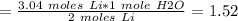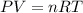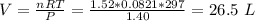Chemistry, 23.12.2019 22:31 terrysizemore666
If 21.3 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 297 kelvin and 1.40 atmospheres? show all of the work used to solve this problem. 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1

Chemistry, 22.06.2019 05:00
Agas can holds 2.0 gal of gasoline. what is this quantity in cubic centimeters?
Answers: 2

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
If 21.3 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at...
Questions




Mathematics, 24.03.2021 03:20



Health, 24.03.2021 03:20

Chemistry, 24.03.2021 03:20

Advanced Placement (AP), 24.03.2021 03:20


Advanced Placement (AP), 24.03.2021 03:20

Social Studies, 24.03.2021 03:20

Mathematics, 24.03.2021 03:20


Mathematics, 24.03.2021 03:20



Mathematics, 24.03.2021 03:20