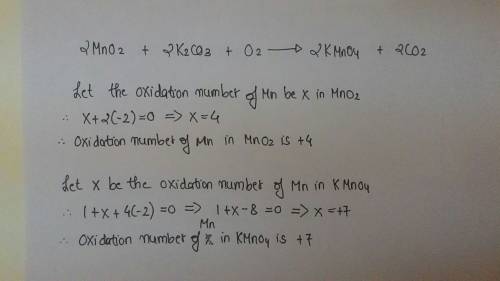Chemistry, 25.08.2019 05:20 marklynr9955
Identify the atom that increases in oxidation number in the following redox reaction. 2mno2 +2k2co3 + o2? 2kmno4 +2co2
a. o
b. mn
c. kd. c

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:30
We just started a new lesson in chemistry and everyone hates it and i dont get it one bit. i hate school. h el p.balanced equationc3h8+5o2-> 3co2+4h2o1.) if you start with 14.8g of propane(c3h8) and 3.44g of oxygen, which is the limiting reactant -check my answer 2.)what mass of excess reagent is left over? 3.)what mass of carbon dioxide can be made? 4.)what mass of water is produced?
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Identify the atom that increases in oxidation number in the following redox reaction. 2mno2 +2k2co3...
Questions

History, 20.07.2019 15:40





History, 20.07.2019 15:40


Physics, 20.07.2019 15:40

Social Studies, 20.07.2019 15:40



Mathematics, 20.07.2019 15:40





Mathematics, 20.07.2019 15:40

Social Studies, 20.07.2019 15:40

English, 20.07.2019 15:40