What are the steps needed when solving problem like this?
a compound of uranium and fluorine...

Chemistry, 14.01.2020 05:31 makaileep7449
What are the steps needed when solving problem like this?
a compound of uranium and fluorine is used to generate uranium for nuclear power plants. the gas can be decomposed to yield 2.09 parts by mass of uranium for every 1 part by mass of fluorine. if the relative mass of a uranium atom is 238 and the relative mass of a fluorine atom is 19, calculate the number of fluorine atoms that are combined with one uranium atom.
search entries or author
filter replies by unread

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2


Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Questions


Mathematics, 27.04.2021 19:20

Computers and Technology, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Social Studies, 27.04.2021 19:20

World Languages, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20





English, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20


Mathematics, 27.04.2021 19:20

Mathematics, 27.04.2021 19:20

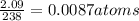
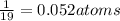
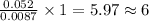 atoms of Fluorine atoms.
atoms of Fluorine atoms.

