
Chemistry, 25.09.2019 11:30 kimlyn58p0wyn0
Kylie has two glasses with equal volumes of pure water. she adds 40 g of sugar to one of the glasses of water and 40 g of salt to the other. when sugar is dissolved in water, it does not change the number of ions present. when salt is dissolved in water, however, the number of ions significantly increases.
which of the following is true about the electrical conductivity of kylie's two solutions?
a)the solution of sugar water will have a higher conductivity than the solution of salt water.
b) pure water will have a higher conductivity than both the sugar water and the salt water.
c) the solution of salt water will have a higher conductivity than the solution of sugar water.
d) the solution of salt water and the solution of sugar water will have the exact same conductivity.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2

You know the right answer?
Kylie has two glasses with equal volumes of pure water. she adds 40 g of sugar to one of the glasses...
Questions

Mathematics, 13.01.2020 05:31


Mathematics, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31

History, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31


Mathematics, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31

Mathematics, 13.01.2020 05:31

Social Studies, 13.01.2020 05:31


Mathematics, 13.01.2020 05:31





Mathematics, 13.01.2020 05:31

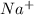 and
and 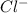 which are electrolytic ions. This occurs because the NaCl is a strong electrolyte in nature as it can get easily dissociated into its ions. Also, solutions of strong electrolytes are observed to be good conductors of electricity as they contain a comparatively high concentration of ions.
which are electrolytic ions. This occurs because the NaCl is a strong electrolyte in nature as it can get easily dissociated into its ions. Also, solutions of strong electrolytes are observed to be good conductors of electricity as they contain a comparatively high concentration of ions.

