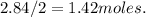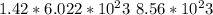13. solid sodium reacts violently with water producing heat, hydrogen gas and sodium hydroxide. how many molecules of hydrogen gas are produced when 65.4 g of sodium are added to water? 2na(s) + 2h2o(l) → 2naoh (aq) + h2(g)(1 point for molar mass of sodium, 1 point for correct mole ratio, 1 point for work, 1 point for correct answer with correct units) (4 points total) *
your answer

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:40
It is important to wear proper protective equipment in lab even when not actively performing experiments because accidents can affect any researcher, even one not working on an experiment. select the best answer from the choices provided
Answers: 3

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 23.06.2019 05:30
According to thomson, the atom is a positively charged cloud with electrons scattered throughout. what would the alpha particles do when they hit the foil if thomson were correct
Answers: 1
You know the right answer?
13. solid sodium reacts violently with water producing heat, hydrogen gas and sodium hydroxide. how...
Questions




Mathematics, 26.10.2019 06:43

Biology, 26.10.2019 06:43

Biology, 26.10.2019 06:43

Mathematics, 26.10.2019 06:43

History, 26.10.2019 06:43


History, 26.10.2019 06:43

Mathematics, 26.10.2019 06:43

English, 26.10.2019 06:43





History, 26.10.2019 06:43

Chemistry, 26.10.2019 06:43

Social Studies, 26.10.2019 06:43