Chemistry, 28.12.2019 14:31 QueenNerdy889
Which statement is true according to the kinetic theory?
molecules of different gases with the same mass and temperature always have the same average density.
molecules of different gases with the same mass and temperature always have the same average volume.
molecules of different gases with the same mass and temperature always have the same pressure.
molecules of different gases with the same mass and temperature always have the same molecular mass.
molecules of different gases with the same mass and temperature always have the same average kinetic energy.
nextreset

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1

You know the right answer?
Which statement is true according to the kinetic theory?
molecules of different gases with th...
molecules of different gases with th...
Questions



Biology, 09.02.2021 16:50









Mathematics, 09.02.2021 16:50


Mathematics, 09.02.2021 16:50

History, 09.02.2021 16:50


Social Studies, 09.02.2021 16:50


Computers and Technology, 09.02.2021 16:50

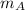 and
and 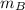 and velocity
and velocity 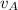 and
and 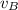 respectively. If
respectively. If 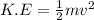 . Then it follows that
. Then it follows that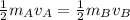 . Since the mass is the same it means the velocity of the two objects should be the same so that they have the same average kinetic energy.
. Since the mass is the same it means the velocity of the two objects should be the same so that they have the same average kinetic energy.