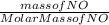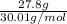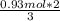Chemistry, 31.08.2019 18:10 jesh0975556
In the following reaction, how many grams of ammonia (nh3) will react with 27.8 grams of nitric oxide (no)? 4nh3 + 6no → 5n2 + 6h2o the molar mass of ammonia is 17.0337 grams and that of nitric oxide is 30.01 grams.
a- 23.7 grams
b- 10.5 grams
c- 73.5 grams
d- 32.7 grams

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
The density of glycerin is 1.26grams/centimeter cubed . how many is this? use the conversion rates of and . express your answer to the correct number of significant figures.
Answers: 1

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
In the following reaction, how many grams of ammonia (nh3) will react with 27.8 grams of nitric oxid...
Questions

Mathematics, 28.07.2021 06:00

Social Studies, 28.07.2021 06:00




English, 28.07.2021 06:00


Mathematics, 28.07.2021 06:00

Mathematics, 28.07.2021 06:00


Mathematics, 28.07.2021 06:00

Mathematics, 28.07.2021 06:00


Mathematics, 28.07.2021 06:10


Chemistry, 28.07.2021 06:10


Mathematics, 28.07.2021 06:10