
You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) + 2al(s)⟶ 2alcl3(aq) + 3cu(s) however, impurities and errors can occur easily. suppose that only 2.86 g of copper is produced. what is the percent yield of the reaction

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) +...
Questions




Computers and Technology, 28.06.2019 17:00



History, 28.06.2019 17:00



Social Studies, 28.06.2019 17:00



English, 28.06.2019 17:00


English, 28.06.2019 17:00



English, 28.06.2019 17:00


Mathematics, 28.06.2019 17:00

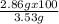 = 81 %
= 81 %

