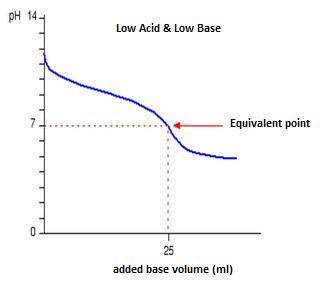
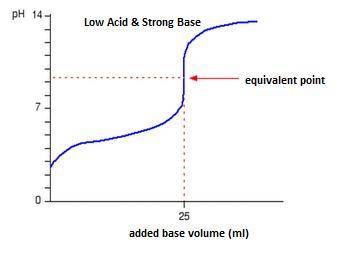
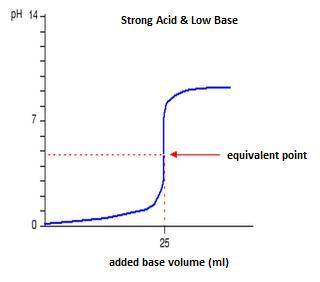
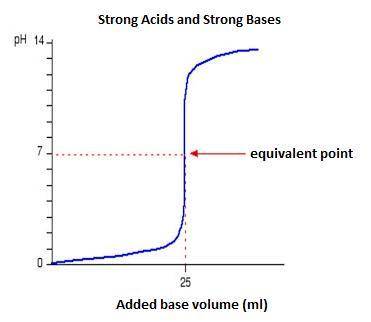
Classify each titration curve as representing a strong acid titrated with a strong base, a strong base titrated with a strong acid, a weak acid titrated with a strong base, a weak base titrated with a strong acid, or a polyprotic acid titrated with a strong base.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 23.06.2019 05:30
How many moles are in 1.26*10^24 particles in significant figures
Answers: 2

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

You know the right answer?
Classify each titration curve as representing a strong acid titrated with a strong base, a strong ba...
Questions

Mathematics, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00


Mathematics, 05.03.2021 19:00


Business, 05.03.2021 19:00

Spanish, 05.03.2021 19:00

Biology, 05.03.2021 19:00



Mathematics, 05.03.2021 19:00


Mathematics, 05.03.2021 19:00

English, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00

Mathematics, 05.03.2021 19:00