
Chemistry, 24.01.2020 08:31 natalie9316
1)describe in a step by step manner how you determined the identity of the two unknowns. discuss both what you determined the unknowns to be and the method that you used to find them.
2)use your knowledge of collision theory to explain the results of your experiments in this laboratory.
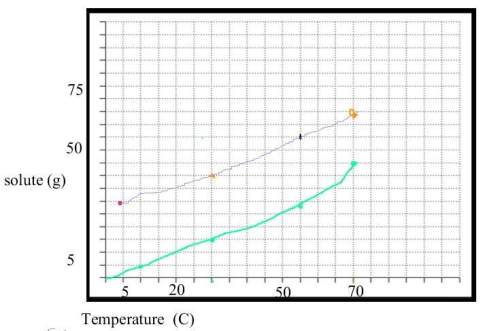

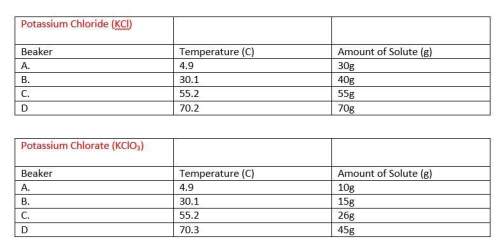


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
1)describe in a step by step manner how you determined the identity of the two unknowns. discuss bot...
Questions

English, 30.03.2021 16:20

History, 30.03.2021 16:20

English, 30.03.2021 16:20

English, 30.03.2021 16:20

Mathematics, 30.03.2021 16:20



Mathematics, 30.03.2021 16:20



History, 30.03.2021 16:20

Mathematics, 30.03.2021 16:20




Mathematics, 30.03.2021 16:20

Mathematics, 30.03.2021 16:20

Mathematics, 30.03.2021 16:20

Spanish, 30.03.2021 16:20

Mathematics, 30.03.2021 16:20



